Insight into alternate bearing — do carbohydrates drive it?

Figure 1—We collected pecan fruiting shoots including current, one-year, and two-year sections, and tested the carbohydrates level of each separately. The shoot samples were collected from fruit set through harvest in 2019 to 2021.
Dr. Mike Smith helped support this theory. Compared to other seed tree crops, this evolutionary trait is more obvious in pecan trees which are within only a few generations from the wild and much more similar to their forebears than other fruit species, and retain the intermediary alternate behavior—showing a more irregular character than the alternate bearing described in other tree crops.
A symptom caught our interest. Even on the same improved cultivar, “masting” is apt to occur in the central pecan region, such as Oklahoma and in parts of Texas, where trees are rarely pruned. In western, southeastern, and some parts of central production regions where trees accept mechanical pruning, trees more likely follow alternate bearing than “masting.”
So, the question is: how does pruning change the tree’s fruiting patterns in masting or alternative bearing? Essentially, mechanical pruning is done to increase the mixture of different types of shoots; in other words, it’s used to increase vegetative growth, producing more carbohydrates.
Flowering and Carbohydrates
Carbohydrate depletion is generally accepted as a strong factor in explaining the phenomenon of alternate bearing in pecans and other tree crops. Carbohydrates provide energy for plant maintenance, growth, defense, and reproduction. Originally proposed by researchers while studying the masting phenomenon, a resource budget model (Prasad and Sakai, 2015) provides a reasonable explanation.
The resource budget model proposes that plants use most carbohydrates with priority for maintenance and growth; flowering and fruiting occur only when the amount of the remaining carbohydrates exceeds a certain threshold level. In other words, crops need some time to accumulate enough energy for flowering and fruiting; it could take them one or more years to enter an “on” year (large production). Thus, it is not surprising that pecan trees sometimes have multiple consecutive “off” years (small production).
Other research in pecans also demonstrated that alternate bearing is associated with the absence and abortion of pistillate flowers, and the development of pistillate flowers may be arrested when carbohydrates are low (Rohla, 2014). For our own study, we hypothesize that overproducing nuts could be an essential factor in shortening the resource budget. A smaller resource budget results in poor flowering in pecans in the current season due to a lack of carbohydrates reserved from the previous season. Here we give four potential reasons:
1) The pecan harvest occurs late— in October and November (Oklahoma)—compared to most other tree crops. A long fruiting period in the previous season could deprive the tree of carbohydrates as well as result in a shortened post-harvest period for carbohydrate accumulation (photosynthesis) and storage.
2) In most other crops, the floral bud primordium and fruiting form synchronously; however, in pecans the pistillate (female) flower differentiates early the following spring. Budbreak and bloom then occur right after flower differentiation in the spring. This flowering habit could exhaust stored carbohydrates.
3) Female pecan flowers normally develop from a mixed or compound bud, which bears on the current year’s new shoot. The new leaves need time to become fully functional, and this process needs more reserved carbohydrates to build biomass (shoot, leaves, flowers).
4) Male flowers are born at the base of the female shoot. As a competing sink (organs use carbohydrates), the male flowers’ bearing location and bloom timing could be a factor inhibiting shoot flush and pistillate flower development.
Based on the above, we hypothesize: insufficient carbohydrates reserved from the previous growth season cause poor pistillate bud differentiation and development, triggering alternate bearing in pecans. Furthermore, the large exhaustion and slow accumulation of carbohydrates could be the reason for the irregular fruiting habit in pecans. There could be a minimum threshold of carbohydrates for pistillate flowering, and trees may need one or more years to achieve this level.
Carbohydrates Observation
Our previous paper in Pecan South’s January 2020 issue introduced the nut growth model to provide guidance when collecting and measuring nut samples from fruit set through harvest. Indeed, when we collected nut samples, we also sampled the whole shoots bearing the same fruits. The shoots include current, one- and two-year sections, as Figure 1 shows. Each section was tested separately for total sugar and starch; we expect to develop a pecan tree carbohydrate budget model from these tests.
Carbohydrate budget varying year to year provides a clue to alternate bearing.
Scientists generally agreed that in the spring before budbreak, carbohydrates stored in the roots are slowly depleted during the winter and the consumption rate increases. Starch stored in the roots degrades and is delivered to shoots as soluble sugars via xylem (wood) sap. Once in the shoot, these sugars are again transformed into starch and stored to support later budbreak and bloom.
Through two years of data in 2019 and 2020, we found that after fruit set in spring, the sugar levels in wood vacillated between the two years. For both ‘Kanza’ and ‘Pawnee’ cultivars, the wood contained approximately 55 to 60 mg/g sugars in June 2019; however, the wood contained only an average of 15 mg/g sugars in June 2020 (Fig. 2 a, b).
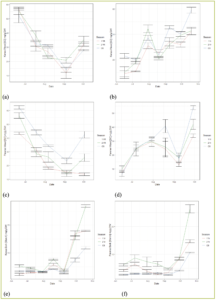
Figure 2—Carbohydrate budget of (a) sugar content in wood of ‘Kanza’ in 2019; (b) sugar content in wood of ‘Kanza’ in 2020; (c) sugar content in wood of ‘Pawnee’ in 2019; (d) sugar content in wood of ‘Pawnee’ in 2020; (e) starch content in bark of ‘Kanza’ in 2019; (f) starch content in bark of ‘Pawnee’ in 2019.
We analyze this phenomenon in two aspects. First, in June, since most leaves still haven’t fully unfolded, most carbohydrates come from last year’s storage. The leftover’s different levels at the early season reveal again that the carbohydrates stored from the previous season could be related to flower differentiation and return bloom.
Moreover, another budget could exist that the trees decide themselves—to guarantee enough carbohydrates for early fruiting, the carbohydrates leftover after bloom should be kept at a higher level.
Research in other tree fruit/nut crops, such as peach (Silva et al., 2014), shows the carbohydrates in shoots decreased from March to June, but in November, all tissues recovered to the levels before March. For both ‘Pawnee’ and ‘Kanza,’ we also observed carbohydrate return (sugar and starch) in the winter season. However, the recovery levels differed between years.
Taking an example of sugar in ‘Kanza’ wood (Fig 2. a, b), the sugar in October 2019 recovered to 28-35 mg/g, while in 2020 it recovered to 40-60 mg/g. The lower sugar level early in 2020 was partially because of the lower storage in 2019, even though we do not have a full picture of carbohydrate data, including levels for November to May.
The difference between the carbohydrate budget pattern in 2019 and 2020 gives us a clue toward understanding masting and alternate bearing carbohydrate partners. Carbohydrate collection does not follow a yearly cycle, which has misled some previous research. In other words, carbohydrate budgeting’s multi-year cycle indicates again that carbohydrates could drive the masting or biennial fruiting pattern. For growers, practical tools to improve photosynthesis or reserved carbohydrates could keep trees in consistent production.
Fruiting and Carbohydrates
In all the tests, across cultivars and years, trees showed extreme sugar exhaustion or requirements at the end of August and in early September (Fig 2 a-d), when nuts transitioned to the dough stage where the embryo is fully developed and oil settled. This finding is entirely consistent with Dr. Maness’ assumption that late-season oil accumulation in the nuts, occurring over three to four weeks, depletes carbohydrate reserves. Later, sugar values returned to a higher level, possibly because of the carbohydrate relocation at the whole tree level, but further research is required.
In addition, many studies support the fact that carbohydrate consumption is autonomous and that shoot carbohydrates stored near flowers/nuts were considered the main source supporting bloom and early fruiting. As stated above, we collected shoots near the fruits at different distances: a current year section that directly bears the fruits; one-year section, the second closest section; and two-year section, the third closest section to the fruits.
Our observation shows the sugar level in the current year’s section is higher than the other sections, especially in ‘Pawnee’ (Fig. 2 c,d), but more starches were stored in two-year-old sections (Fig. 2e,f). This result created a vivid picture of the functions of different sections in the shoot. The current shoot, which bears both fruits and shoots, is the sugar station that continuously receives carbohydrates from leaves and provides “food” to the fruits. The older shoots act as backup, taking charge to reserve carbohydrates for the following years. However, we still do not fully understand the distance of the carbohydrates’ translocation from older shoots to current year shoots and vice versa. This information importantly relates to the efficiency of pruning strategies. How can we prune trees to maximize carbohydrate reservation? We will further explore this question in future research.
Does pecan have a lower starch level?
Another interesting finding we would like to share is that through thousands of samples testing in both pistachio and pecans, an obviously lower starch level appeared in pecan trees. The starch level in pecan was approximately three to four times lower than starch in pistachio throughout the season. As we know, pistachio tightly follows an alternate bearing pattern, and the pecan trees we used are more apt to the masting pattern.
This caused us to think about another factor: could the 2-plus year cycle in pecans be driven by a lower starch level? Do the mechanically pruned trees that follow alternate bearing have a higher level of starch than the rarely pruned (masting) trees?
We will study this topic further in collaboration with different states in the hopes that these results will provide us with an answer to proper tree pruning management.
This carbohydrates study is intended to bolster the many orchard management applications already in play—such as fruit thinning, pruning, and plant growth regulators to mitigate alternate bearing. These applications all play a role in helping trees avoid the wasteful consumption of carbohydrates and increase carbohydrate storage. We will continue to update our current research results in Pecan South.

