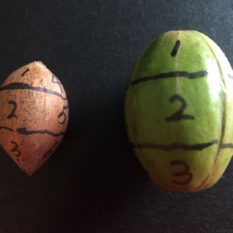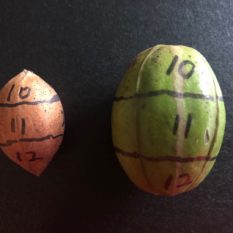OSU Study Links Nut Firmness and Weevil Infection

Measuring pecan shell firmness using force gauge with a 2mm probe needle.
The pecan weevil Curculio caryae is the most severe arthropod pest of pecan in Oklahoma, resulting in extremely erratic pecan production predictability (Smith and Mulder, 2009). Mulder et al. (2012) cited that pecan weevils cause three types of damage to pecan: early in the season, weevils cause shell punctures in young nuts during the water stage resulting in nuts that fall from the tree; later in the season, weevils lay eggs in pecans causing ovipositional damage, which ultimately results in the final form of damage caused by pecans, which is larva hatching inside the shell and feeding on the kernel. Our current focus is to determine at which point and how shell hardening—or nut firmness—restricts weevil infection and egg laying in order to limit ovipositional damage.
Female pecan flowers have three functional structures: stigma, style, and ovary. The wall of the ovary has three layers: exocarp, mesocarp, and endocarp. The ovary wall of pecans is fused to the calyx. The endocarp develops into the shell, which encloses the embryo, while the left part of the ovary wall will develop into the shuck.
Nuts of ‘Kanza’ cultivars were collected weekly from the experimental orchard at the OSU Cimarron Valley Research Station in Perkins, Oklahoma, and the firmness of shells tested using a force gauge (Imada Co., LTD) with a 2mm diameter probe needle. There were six nuts with visible weevil damage in late August, which are likewise labeled in Figure 2. Figure 2 also shows the nuts divided into 12 sections, the firmness of each section was evaluated, and sections with weevil damage were marked.
Fruit morphology research shows that female pecan flowers consist of two carpels with obvious connection lines (sutures) visible on the shell (Fig 2b). There are four surfaces on the shuck that are naturally separated by extrusion lines. An observation of interest is that for many pecan cultivars, such as ‘Pawnee’ and ‘Kanza,’ there is a discolored line of older tissue on the shuck surface (Fig 2a) appearing on three surfaces with the fourth surface left with no line or only a very faint trace (Fig 2b). There is no known explanation for this phenomenon, but research on English walnut Juglans regia states that a difference between initiation of the two carpels could provide a clue toward understanding the discrepancy of the discolored lines (Donald Shuhart, 1932).
A carpel is the female reproductive part of the flower, interpreted as modified leaves that bear structures called ovules, inside which the egg cells ultimately form the ovary, style, and stigma. The two surfaces of the shell with suture are underneath the two shuck surfaces adjacent to the surface without a discolored line. In addition, the nut has two ends (Fig 2a)—the base end connected to the fruit stem and the distal end that previously set the flower stigma. This difference provides us the possibility to label the 12 sections on nuts without opening the kernel. Holding the nut with the base up and distal end down, and moving in a clockwise direction from the clear shuck surface, the base end is marked 1, 4, 7, 10; the middle is marked 2, 5, 8, 11; the distal end is marked 3, 6, 9, 12 (Fig 2). Noting that the nuts harvested in August were collected earlier than the nuts used in Figure 2 because the shuck and shell are connected and not easily separated for illustration in early stages.
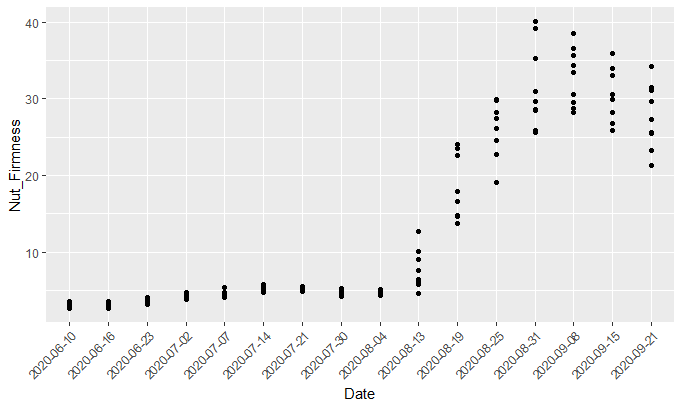
Figure 3: Shell firmness (sections 2 or 8) of Kanza nuts collected from early fruit set July through September in 2020.
We found through our research that the curve of pecan firmness (Fig. 3) was low at the beginning and rarely increased until the end of July. Shell-hardening increased quickly throughout August and firmness remained steady in September. In Oklahoma, August represents the typical time when nuts go through the three stages: water, gel, and dough stages. Though they were all in the dough stage, the values varied among individual nuts, which provides us the space to infer that weevils could oviposit into nuts with weaker firmness measurements. Our records show that on August 30 all ‘Kanza’ nuts had entered the dough stage. Our measuring of the firmness will continue throughout the harvest, and we will keep you updated on this research in subsequent issues of Pecan South.
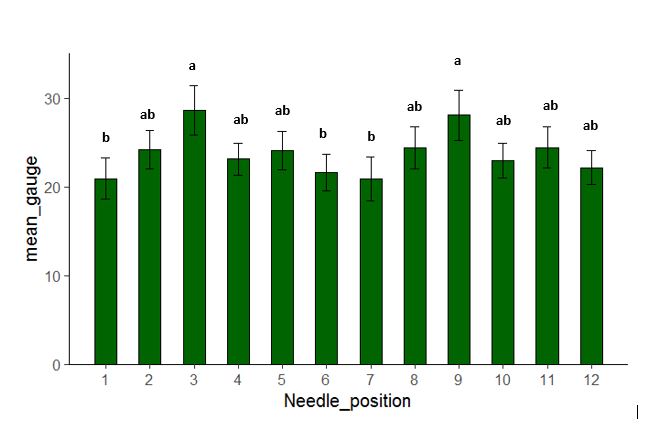
Figure 4: The average values of nut firmness (lb/2mm2) of the 12 sections of the six nuts on which the asterisk (*) indicates sections with weevil damage. Values on sections 3 and 9 are significantly higher than sections 1, 6 and 7, while other sections are not significantly different from one another.
Figure 4 shows the average values of nut firmness (lb/2 mm2) of the 12 sections of the six nuts on which the star (*) indicates the section with weevil damage. It is obvious that the firmness value of each section varies. The values in sections 3 and 9 are significantly higher than sections 1, 6, and 7, while other sections are not significantly different from one another. Sections 3 and 9 are located on the distal non-sutured end; section 6 is at the distal suture end (notice that the firmness of other distal suture ends with section 12 is also low in value). Smith and Mulder (2009) stated that weevil punctured holes appear most often on the distal end, and our research appears to support this statement. However, our research provides additional information: sutures are the weakest point on the shell. This observation may be where further research could reveal that puncture holes may be found more frequently near the suture.
Continuing with Figure 4, all of the five sections with weevil damage are on the sections showing the lowest nut firmness (1, 6, 7), except for section 11 which has a moderate nut firmness. This finding suggests that weevils select the location where oviposition could be dependent on lower nut firmness values. This preliminary research provides the possibility for future research exploring how to restrict weevil activity based not on each nut’s weakest sectional firmness but on the growth timeline.
A large number of nuts would be required to further prove this phenomenon. However, this study leads us to consider weevil behavior and nut growth. There is also potential to provide information on managing weevil activity through the prediction of nut growth and maturity. We hypothesize that the level of weevil activity is determined based on shell hardness.